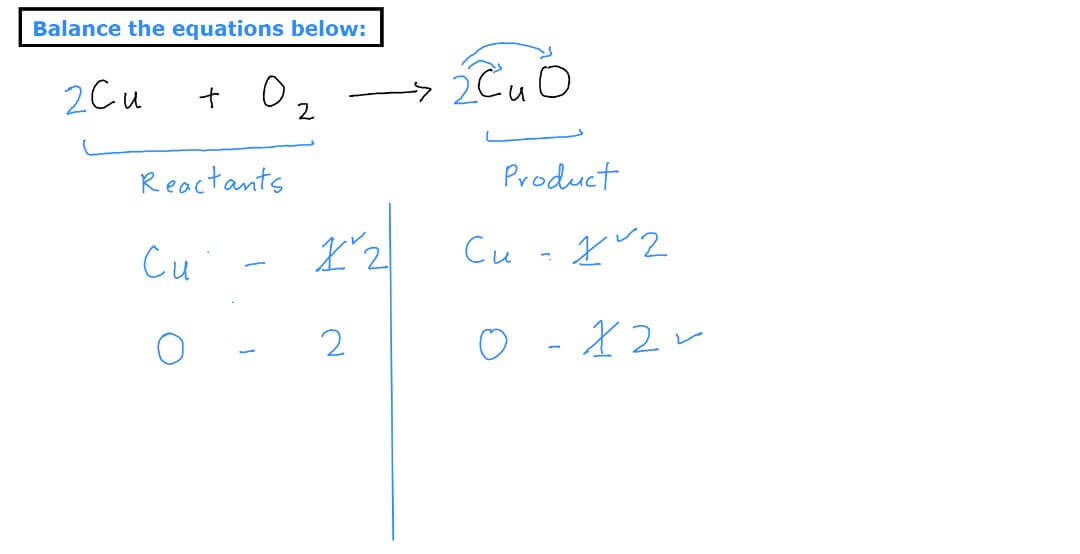
How to balance a chemical equation:
To write a balanced equation, you need to first identify the reactants (the starting materials) and the products (the resulting substances) of the reaction. Next, write the chemical formulas for each reactant and product. Then, use coefficients (numbers written in front of the chemical formulas) to balance the number of atoms of each element on both sides of the equation.
For example, the balanced equation for the reaction of hydrogen gas (H2) and oxygen gas (O2) to form water (H2O) would be:
2H2 + O2 -> 2H2O
Here, the coefficients “2” in front of the H2 and H2O on the reactant and product side ensure that there are the same number of hydrogen atoms on both sides of the equation.
It’s also important to make sure that the equation is balanced by checking the number of atoms on the LHS & RHS and if needed use the coefficients.