Paper chromatography is a common laboratory technique used to separate and analyze the components of a mixture.
Here are the steps to perform it at home:
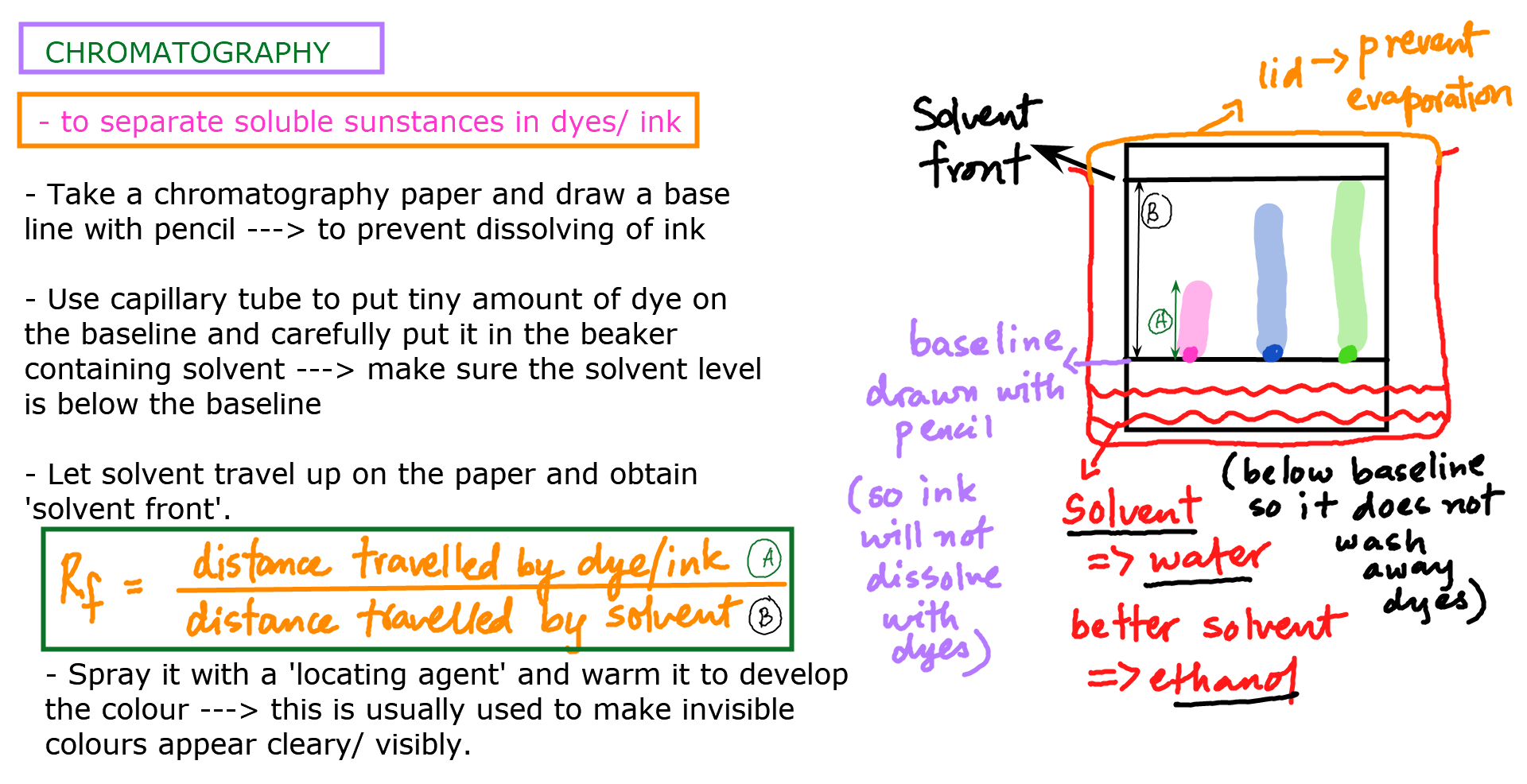
Steps to carry out paper chromatography experiment at home:
Cut a piece of chromatography paper and place a small dot of the mixture to be separated near the bottom of the paper.
Fold the paper and place it in a container filled with a solvent, such as water or alcohol, which will travel up the paper by capillary action.
Observe the solvent as it moves up the paper. Different components of the mixture will move at different rates, separating from each other as the solvent moves.
Once the solvent reaches the top of the paper, remove the paper from the container and allow it to air dry.
Compare the resulting separated components to a reference chart or to known samples to identify each component.
Safety precautions:
Safety precautions such as wearing gloves and working in a well-ventilated area should be taken when working with solvents.
The Rf value (retention factor) is a measure of how far a particular component of a mixture has traveled up a chromatography paper compared to the solvent front. The Rf value can be used to identify the components of the mixture.
How to find Rf value?
Here’s how to find the Rf value:
Measure the distance from the starting point to the center of the spot or band representing the component of interest (distance traveled by the solute).
Measure the distance from the starting point to the solvent front (distance traveled by the solvent).
Divide the distance traveled by the solute by the distance traveled by the solvent to get the Rf value:
Rf = distance traveled by (dye) solute (divided by) the solvent front – distance traveled by solvent
Note that the Rf value will depend on the type of solvent used and the conditions of the chromatography experiment. It is a characteristic of a particular solute under specific conditions and can be used to identify unknown components by comparing their Rf values to known values for similar substances.
Uses of Chromatography:
Chromatography is a widely used technique in many fields, including chemistry, biochemistry, and forensics, for separating and analyzing mixtures of chemical compounds. Some of the key uses of chromatography are:
Analysis of biological samples: Chromatography is used to isolate and identify proteins, lipids, and other biomolecules from complex biological samples, such as blood, urine, and tissues.
Environmental analysis: Chromatography is used to detect and measure pollutants in air, water, and soil samples.
Food analysis: Chromatography is used to identify and quantify additives, preservatives, and contaminants in food products.
Drug analysis: Chromatography is used to identify and quantify drugs in biological samples, such as blood, urine, and hair, to monitor drug abuse and compliance with medication regimens.
Forensics: Chromatography is used to identify and quantify drugs, poisons, and other toxic substances in forensic samples, such as blood, urine, and tissue.
Quality control: Chromatography is used in the pharmaceutical and chemical industries to test the purity and composition of raw materials, intermediates, and final products.
Synthesis of chemicals: Chromatography is used to purify and isolate the desired product from reaction mixtures, improving the overall yield and quality of the synthesized chemical.
Overall, chromatography is a versatile and powerful tool for separating, identifying, and quantifying complex mixtures, providing critical information for a wide range of applications in science, medicine, and industry.